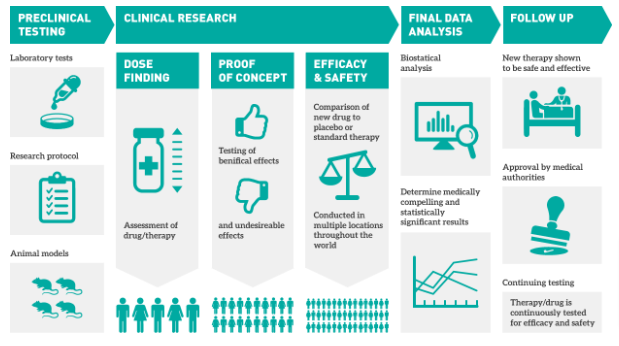
Before patients receive innovative treatment, drugs undergo clinical trials. According to PhRMA’s calculations, one trial lasts an average of 6-7 years and costs $2.6 billion. Due to various issues, pharmaceutical companies delay 80% of releases. Clinical Leader says that 30% of projects are closed during the last phases of testing. All this ruins clinicians’ efforts. To avoid this, healthcare software development companies build clinical trial software. It solves such problems efficiently. Let’s look at key types of this software – EDC, CTMS, RTSM, and IRT.
Why do clinical trials fail?
Californian researcher David B. Fogel cites five main reasons for the failure of clinical trials:
- Drugs are not effective enough because of imperfect design, limited sampling, and incomplete data collection.
- Physicians and patients have different notions of side effects.
- Initial cost estimates differ from actual trial costs, and investors are not willing to overpay for these services.
- There are not enough patient volunteers because of poor recruitment, registration, and retention.
- Studies are geographically limited. Trials involve residents of local areas who can come to centers regularly.
Clinical trial software can eliminate or partially ease these problems.
CTMS software: key functions and features
Clinical trial management software (CTMS) is a mobile or web-based app that allows medical, pharmaceutical, or biotechnology companies to manage clinical processes. Such a system offers researchers useful tools to interact with patients and adhere to clinical trial protocols.
CTMS helps researchers
- manage trials by assigning tasks, tracking team performance, and monitoring study duration;
- issue invoices and monitor financial accountability;
- provide access to other team members. At each stage of a clinical trial, participants carry out different tasks. They need access to separate interfaces and templates;
- compile research results into an overall report and send it to investors and other stakeholders.
Since the information CTMS solutions collect is strictly confidential, such a system includes an access control function. Only employees of a research center can work with this data. You can copy information important for research to the cloud and can easily restore it after a failure. You can upload multi-format files to a CTMS (documents, images, and diagrams). Created reports are stored locally or in the cloud.
With CTMS software, researchers do not waste time writing reports manually. Experts find and correct errors before they lead to the closure of clinical trials.
Electronic data capture (EDC)
An electronic data capture system (EDC) is a web-based platform that stores information about participants in clinical trials. Specialists take down patients’ medical data on paper and then transfer it to the EDC. Electronic records are used in simple and complex trials because they make it easier to collect and store information.
Three key groups use electronic data capture systems:
- medical institutions turn to them for statistics;
- with their help, investors track research progress;
- thanks to EDC software, research centers exchange reports with their stakeholders.
An EDC system has a cloud-based server, making the app easily scalable as the volume of data grows. Such a platform has built-in role-based access control: each user has a unique name and set of permissions. The platform shows who worked in the system, what they did, and why they changed a record. You can create reports in the app and export them as tables, CSV files, or other formats.
EDC systems ensure that medical data is stored securely. Information is available in real time, so you can effortlessly search and filter it. Thanks to such apps, managers, sponsors, and researchers enjoy up-to-date information and understand the progress of trials in the same way.
Randomization and trial supply management platforms (RTSM)
It is difficult to select participants for complex clinical trials manually. Research centers either recruit many patients who do not fit the characteristics or lack enough volunteers.
RTSM software controls the assignment of people to experimental groups, keeps track of drug inventories in stock, and dispenses drugs to subjects. The system groups patients by gender, age, bad habits, chronic diseases, and other parameters. It forms a cohort according to the right criteria and different scenarios, calculates the drug dosage for each patient, and controls medication dispensing.
An app describes a drug under study in detail and helps track patients through incoming alerts. With the randomization software, researchers accurately assign patients to treatment groups. They make sure there is enough medication for all volunteers for the duration of a trial.

Interactive response technology platforms (IRT)
IRT platforms are software solutions similar to RTSMs in their functionality. Their primary focus is drug randomization and supply control. Trialists use such apps to order the right amount of medication, think through economical use options, and monitor medication dispensing.
IRT software establishes communication between manufacturers, sponsors, researchers, and shipping companies. Such a system allows specialists to calculate how much medication is necessary for the duration of a study, considering dosage and expiration date. Scientists order the right medicine in advance to agree with the manufacturer on how the drugs will be produced and packaged. As a result, there is no oversupply of drugs, and companies don’t lose money.
Conclusion
Clinical trial software helps you rationally organize, set up, and conduct trials. All the processes are under control. Managers find and fix problems in time, and the cost of trials does not go beyond the budget. CTMS, EDC, IRT, RTSM, and other platforms connect patients, researchers, and investors and optimize clinical trials.
To build a custom scalable secure platform, turn to an experienced healthcare software development company such as Andersen. Such an IT partner will find out the needs of a research group, help to implement the necessary functionality, and comply with FDA and HIPAA requirements.






